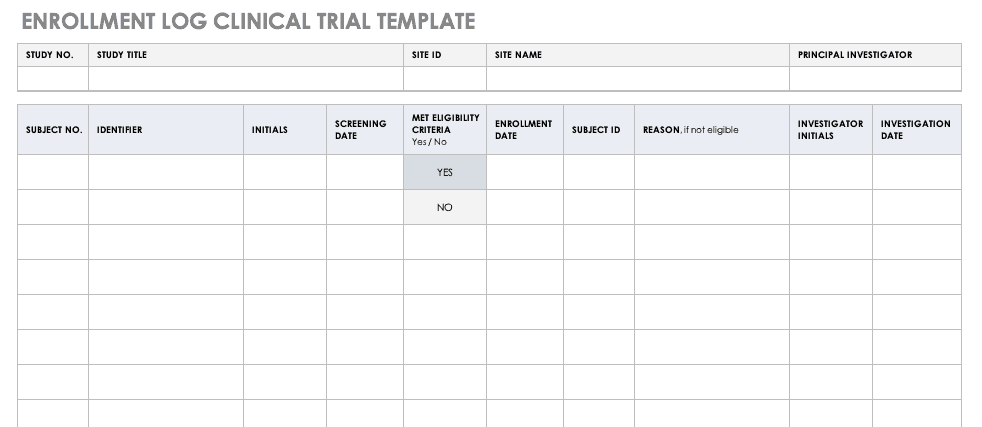
Typical example for a temperature log Essential documents (d) Verifying... | Download Scientific Diagram
Trial Master File / Investigator Site File Index Clinical Trials of Investigational Medicinal Products

Typical example for a temperature log Essential documents (d) Verifying... | Download Scientific Diagram

Typical example for a temperature log Essential documents (d) Verifying... | Download Scientific Diagram

Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial - The Lancet Infectious Diseases

Typical example for a temperature log Essential documents (d) Verifying... | Download Scientific Diagram

OCR Industry Sponsored Study Guide | Office of Clinical Research | Perelman School of Medicine at the University of Pennsylvania
Purpose: To define departmental responsibilities for the safe and accurate handling of investigational drugs. A. The Pharmacy

Typical example for a temperature log Essential documents (d) Verifying... | Download Scientific Diagram

Temperature Monitoring in Clinical Trials in a Risk-Based Regulatory Landscape - Clinical Trials Arena

We are at the forefront of COVID-19 research' — a day in the life of a clinical trials pharmacist - The Pharmaceutical Journal
STANDARD OPERATING PROCEDURE FOR TEMPERATURE MONITORING OF SOP NUMBER: Fife R&D SOP 43 AUTHOR: Jennifer Tait ISSUE DATE: 25/